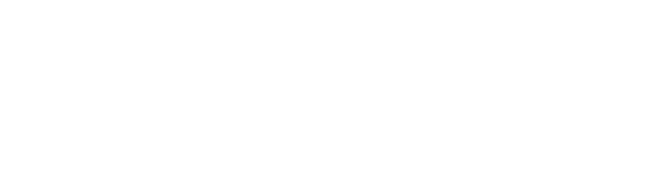Most modern societies place a high value on truth and honesty—but people can’t seem to resist falsehoods, from little white lies to vast conspiracy theories.
How to prevent the next pandemic

5 posts based on my book about avoiding another global outbreak.

Let’s make this the last pandemic
My new book is all about how we eliminate the pandemic as a threat to humanity.
Meet the GERM team
Creating the GERM team is one of the most important things we can do to prevent the next pandemic.
Vaccinate the world in six months
To prevent pandemics, we need to be able to do it. Here's how.
She helped change vaccines forever
Long before most of us heard of mRNA vaccines, this hero saw their potential to save lives.
3 things we can do right now
If we’re going to make COVID-19 the last pandemic, the world needs to get to work right away on these key areas.
Endgame
Let’s make this the last pandemic
My new book is all about how we eliminate the pandemic as a threat to humanity.
The great epidemiologist Larry Brilliant once said that “outbreaks are inevitable, but pandemics are optional.” I thought about this quote and what it reveals about the COVID-19 pandemic often while I was working on my new book.
On the one hand, it’s disheartening to imagine how much loss and suffering could’ve been avoided if we’d only made better choices. We are now more than two years into the pandemic. The world did not prioritize global health until it was too late, and the result has been catastrophic. Countries failed to prepare for pandemics, rich countries reduced funding for R&D, and most governments failed to strengthen their health systems. Although we’re finally reaching the light at the end of the tunnel, COVID still kills several thousand people every day.

On the other hand, Dr. Brilliant’s quote makes me feel hopeful. No one wants to live through this again—and we don’t have to. Outbreaks are inevitable, but pandemics are optional. The world doesn’t need to live in fear of the next pandemic. If we make key investments that benefit everyone, COVID-19 could be the last pandemic ever.
This idea is what my book, How to Prevent the Next Pandemic , is all about. I’ve been part of the effort to stop COVID since the early days of the outbreak, working together with experts from inside and out of the Gates Foundation who have been fighting infectious diseases for decades. I’m excited to share what I've learned along the way, because our experience with COVID gives us a clear pathway for how to be ready next time.
So, how do we do it? In my book, I explain the steps we need to take to get ready. Together, they add up to a plan for eliminating the pandemic as a threat to humanity. These steps—alongside the remarkable progress we’ve already made over the last two years in creating new tools and understanding infectious diseases—will reduce the chance that anyone has to live through another COVID.
Imagine a scenario like this: A concerning outbreak is rapidly identified by local public health agencies, which function effectively in even the world’s poorest countries. Anything out of the ordinary is shared with scientists for study, and the information is uploaded to a global database monitored by a dedicated team.
If a threat is detected, governments sound the alarm and initiate public recommendations for travel, social distancing, and emergency planning. They start using the blunt tools that are already on hand, such as quarantines, antivirals that protect against almost any strain, and tests that can be performed anywhere.
If this isn’t sufficient, then the world’s innovators immediately get to work developing new tests, treatments, and vaccines. Diagnostics in particular ramp up extremely fast so that large numbers of people can be tested in a short time. New drugs and vaccines are approved quickly, because we’ve agreed ahead of time on how to run trials safely and share the results. Once they’re ready to go into production, manufacturing gears up right away because factories are already in place and approved.
No one gets left behind, because we’ve already worked out how to rapidly make enough vaccines for everyone. Everything gets where it’s supposed to, when it’s supposed to, because we’ve set up systems to get products delivered all the way to the patient. Communications about the situation are clear and avoid panic.
And this all happens quickly. The goal is to contain outbreaks within the first 100 days before they ever have the chance to spread around the world. If we had stopped the COVID pandemic before 100 days, we could’ve saved over 98 percent of the lives lost.

I hope people who read the book come away with a sense that ending the threat of pandemics forever is a realistic, achievable, and essential goal. I believe this is something that everyone—whether you’re an epidemiologist, a policymaker, or just someone who’s exhausted from the last two years–should care about.
The best part is we have an opportunity to not just stop things from getting worse but to make them better. Even when we’re not facing an active outbreak, the steps we can take to prevent the next pandemic will also make people healthier, save lives, and shrink the health gap between the rich and the poor. The tools that stop an outbreak can also help us find and treat more HIV cases. They can protect more children from deadly diseases like malaria, and they can give more people around the world access to high quality care.
Shrinking the health gap was the life’s work of my friend Paul Farmer, who tragically died in his sleep in February. That’s why I’m dedicating my proceeds from this book to his organization Partners in Health, which provides amazing health care to people in some of the poorest countries in the world. I will miss Paul deeply, but I am comforted by the knowledge that his influence will be felt for decades to come.
If there’s one thing the world has learned over the last two years, it’s that we can’t keep living with the threat of another variant—or another pathogen—hanging over our heads. This is a pivotal moment. There is more momentum than ever before to stop pandemics forever. No one who lived through COVID will ever forget it. Just like a war can change the way a generation looks at the world, COVID has changed the way we see the world.
Although it may not always feel like it, we have made tremendous progress over the last two years. New tools will let us respond faster next time, and new capabilities have made us better prepared to fight deadly pathogens. The world wasn’t ready for COVID, but we can choose to be ready next time.
The outbreak squad
Meet the GERM team
Creating the GERM team is one of the most important things we can do to prevent the next pandemic.
At the beginning of the movie Outbreak, there’s a scene where three government virologists arrive by helicopter at a remote village. Most of the village has recently died from Ebola-like symptoms. Wearing protective moon suits while triumphant music plays in the background, our heroes immediately get to work trying to contain the threat before it hurts anyone else. It’s an inspiring scene.
Unfortunately, it’s pure Hollywood fiction.
A full-time team like this doesn’t exist in real life—yet. I’m hoping this changes soon, because it is one of the most important things we can do to prevent the next pandemic.
Today, there are many organizations that work hard to respond to a major epidemic, but their efforts are largely dependent on volunteers. The best known is the Global Outbreak Alert and Response Network, or GOARN, which does heroic work but doesn’t have the staffing, funding, or global mandate to tackle every threat.
We need a permanent organization of experts who are fully paid and prepared to mount a coordinated response to a dangerous outbreak at any time. In my book, I propose that we call this group the GERM—Global Epidemic Response and Mobilization—team.
The GERM team would be made up of people from all over the world who have a wide range of expertise: epidemiology, genetics, data systems, diplomacy, rapid response, logistics, computer modeling, communications, and more. When they aren’t actively working in the field, most of them would call individual countries’ public health agencies home base, though some would sit in the WHO’s regional offices and at its headquarters in Geneva. (I talked about GERM at length in my TED talk last month.)
It's important that GERM have a diverse workforce. The team is going to serve the entire world—it only makes sense that its members reflect the experiences and backgrounds of the people they’re going to work with. Ideally, GERM would have a high number of local experts from countries at a higher risk of outbreak, and outsiders would only show up when necessary and when the in-country team requests help.
Here’s how a GERM response would work: The team’s disease monitoring experts would look for potential outbreaks. Once it spots one, GERM should have the ability to declare an outbreak and work with national governments and the World Bank to raise money for the response very quickly. Product-development experts would advise governments and companies on the highest-priority drugs and vaccines. People who understand computer modeling would coordinate the work of modelers around the world. And the team would help create and coordinate responses, such as how and when to implement border closures and recommend mask use.
But GERM’s response to an active outbreak is only one part of their work.
The team’s most important job is helping to run outbreak response exercises that test whether the world is ready for the next major outbreak. Militaries regularly run war games to evaluate their readiness—we should do the same with disease threats. In most countries, these exercises can be run by local public health and military leaders, with GERM acting as an advisor and reviewer. For some low-income countries, the world should invest in building this capacity and lend resources as needed.
You can learn more about what these germ games would look like by reading chapter 7 of How to Prevent the Next Pandemic, which is available as a free download for all Gates Notes Insiders.
The GERM team would also be responsible for developing a checklist for pandemic preparedness, similar to the ones that airplane pilots follow before every takeoff and many surgeons now use during an operation. A checklist sounds like such an obvious tool, but very few places had a plan like this in place when COVID hit. A GERM-developed checklist could be used anywhere and help make sure that governments are ready with an efficient and effective response.
But GERM’s impact won’t be limited to stopping pandemics. The group will improve overall health around the world, especially in the poorest countries.
Emerging diseases will always be their top priority, but when there isn’t an active pandemic threat, the team will keep their skills sharp by helping out with deadly diseases like polio and malaria. For example, they could work alongside public health workers in Nigeria to help distribute millions of doses of the oral polio vaccine every year to keep the country polio-free. This would both save a lot of kids from needless suffering and help the GERM team build relationships with communities they will need if an outbreak strikes. Now that’s what I call getting your money’s worth!

Running GERM will cost the world around $1 billion a year to cover salaries for the force of 3,000 people we’d need, plus equipment, travel, and other expenses—money that would come from governments. The work would be coordinated by the WHO, the only group that can give it global credibility, and it needs to be accountable to the public.
When Hollywood gets something wrong, the result is usually pretty silly and unrealistic. But movies like Outbreak nailed it when they imagined a global disease-fighting team who is ready to respond to a crisis on a moment’s notice. If we’re going to make sure that COVID-19 is the last pandemic, we need the GERM team.
Everyone, everywhere
Vaccinate the world in six months
To prevent pandemics, we need to be able to do it. Here's how.
The greatest medical breakthrough of this pandemic—and surely one of the most important in decades—is the creation of COVID-19 vaccines. One study found that in their first year, they saved more than 1 million lives and prevented 10 million hospitalizations in the U.S. alone. The number of deaths averted around the world is of course far higher. It’s horrifying to think what COVID-19 would be doing to humanity if it weren’t for vaccines.
The world has a lot to be proud of in the creation and delivery of these vaccines. Scientists have never developed one nearly as quickly as they did in 2020, and the governments of the world have never run immunization campaigns that were as fast and as far-reaching as the ones that took place in 2021.

But there are also serious problems that we need to solve before the next potential pandemic comes along. One is the huge inequity in who has been vaccinated and who has not. It is both unjust and unwise to give a third shot to a healthy 25-year-old in a rich country before a 75-year-old cancer survivor in a poor country gets her first shot.
Another concern is that the speed with which vaccines were created was only partly a matter of skill and diligence. It was also a matter of luck.
Because coronaviruses had already caused two previous outbreaks (SARS and MERS), scientists had learned quite a lot about the structure of the virus. In particular, they had identified its characteristic spike protein—the tips on the crownlike virus you’ve seen a dozen pictures of—as a potential target for vaccines. When it came time to create new vaccines, they had a sense of what part of the virus was most vulnerable to attack.
In the next outbreak, we may not be so lucky. It could be caused by a virus that scientists haven’t studied as closely, or by one they’ve never seen at all.
This is why the world needs to adopt a serious plan for developing, manufacturing, and distributing new vaccines to prevent another pandemic. The manufacturing alone is a huge challenge: To prevent the inequities we’ve seen in COVID-19, the world needs to be ready to produce enough vaccines for everyone on the planet within six months of discovering a new pathogen. That’s 8 billion doses for a single-dose vaccine, and 16 billion for a two-dose version. In a typical year, around 5 billion or 6 billion doses are produced—that’s all vaccines combined.
The plan needs to cover four steps, starting with accelerating the invention of new vaccines.
During the pandemic, the process of creating a new vaccine got a huge boost (no pun intended). Typically, the process involves a lot of trial and error: Scientists spend years identifying weak spots in the virus and trying to identify vaccine candidates that would teach the immune system to attack them.
The creation of the first mRNA vaccines during the COVID-19 pandemic was a big step forward. They work by delivering genetic code to your body that instructs it to make shapes that look like the weak part of the virus. Your immune system notices that those shapes are foreign and sets out to attack them. Once it does, it remembers what the shapes looked like and will attack them the next time they show up. That’s what makes you immune.
One reason mRNA vaccines were so revolutionary is that they’re easily adapted for different pathogens. Once the weak spot of a virus has been identified—a process made much easier by recent advances in mapping viral genomes—it’s simply a matter of changing the genetic code in the vaccine so that it tells your body to make a new shape. This can be done in a matter of days.
As a result, the development of new vaccines will be exponentially faster—as long as researchers have the same deep understanding of future pathogens as they did of coronaviruses. So it is imperative to invest in basic research on a wider array of known viruses and other pathogens, so we understand as much as possible before the next outbreak.
Once a vaccine has been invented, the second step is to test it and get it approved for use in humans. Typically, it takes years to run all the trials necessary to prove that a vaccine is safe and effective—including time spent recruiting tens of thousands of volunteers. Assuming the vaccine proves out, it can take another year to get it authorized by the WHO and the relevant government agencies.
But when an outbreak is threatening to go global, we won’t have years. So we need ways to speed up the process without sacrificing the safety and effectiveness that people have come to expect from vaccines.

The world should build on models like the RECOVERY trial in the U.K. It set up protocols for running drug trials in advance and built infrastructure that made it much easier to get started once COVID hit. In addition, the agencies that regulate vaccines need to agree ahead of time on how volunteers will be enrolled in trials and on the software tools that will enable people around the world to sign up as soon as the disease strikes. And by connecting diagnostic tests to the trial system, we can automatically suggest to doctors that their patients should join a trial if they’re eligible.
The third step, once a vaccine has been approved for use in humans, is to make enough of it fast enough to stop the outbreak. Ending a relatively small outbreak might require hundreds of thousands of doses of a new vaccine, which is not hard to make. (The world already produces more than 5 billion doses of vaccines every year.) But countries need to be prepared for the worst—another big outbreak in which everyone needs to be vaccinated—so we must be ready to produce as many as 8 billion or even 16 billion, roughly triple the amount manufactured in a typical year.
During COVID-19, the closest thing to a breakthrough in manufacturing vaccines was the proliferation of second-source deals. These are agreements in which a company that invented a vaccine agrees to let other companies use their factories to make it. (Picture Honda Accords rolling off the line of a Ford facility.)
It’s hard to overstate the impact of second-source deals during COVID-19. In less than two years, a single manufacturer, AstraZeneca, signed second-source deals involving 25 factories in 15 countries. (AZ also agreed to forgo its profits on the COVID vaccine.) Novavax also signed one with Serum Institute of India—leading to a COVID-19 vaccine now being used in many countries—and Johnson & Johnson signed one with the Indian company Biological E. Limited and the South African firm Aspen Pharmacare. All told, second-source deals led to the production of billions of additional COVID vaccine doses.
In the future, such deals could be done even faster if companies that have them now can maintain their relationships with one another so they can hit the ground running during the next outbreak.
mRNA vaccines could also help speed up manufacturing. Many of the conventional ways to make vaccines are quite complex, so it can take a lot of time to transfer the technology and know-how from one company to another. But because the basic approach to mRNA is pretty much the same—you just swap out your old mRNA for the new one and make sure the lipid is made the right way—it should be easier to transfer between companies. There are also some new modular technologies in the pipeline that, if they prove out, will make it cheaper and easier to build and run factories that can be adapted to make different vaccines.
Finally, the fourth step in the world’s plan should be to make sure that new vaccines reach everyone who needs them—including people who live in low-income countries. In 2021, only 8 percent of people in those countries received at least one dose of a COVID-19 vaccine, while more than half of the world’s population did.
So how can the world make sure that doesn’t happen in future outbreaks?
One key is to take on the problem of vaccine hesitancy. Check out this video about how, by dealing with rumors and myths, one community in India increased its COVID-19 vaccination rate by a factor of five and created a model that other communities are now taking up:
Another key is to make sure it’s possible to manufacture enough vaccines that supply is not a limiting factor, as it was during much of 2021. Another is to make sure that vaccines are affordable for every country. Organizations like COVAX have helped with that during COVID-19. It also helps to work with manufacturers in developing countries to design new vaccines that are much cheaper to produce than existing ones. This is how the price of the pentavalent vaccine, which protects against five debilitating and deadly diseases, dropped from $3.50 per dose to less than $1 a dose—which in turn allowed the number of children who get it every year to increase by more than 16 times since 2005.
There are also a lot of innovations that make it easier to deliver vaccines. For example, auto-disable syringes have a built-in safety mechanism so health workers can’t accidentally poke themselves or use them more than once. New coolers can keep vaccines at the right temperature for longer. Advanced methods for delivering vaccines, such as replacing the needle and syringe with a small patch containing micro-needles—picture something that looks superficially like the nicotine patches that people use to stop smoking—will also help.
With these advances, it will be possible to achieve something amazing beyond preventing pandemics: eradicating entire families of pathogens. The world could rid itself of all coronaviruses, for example, or even all influenza viruses. A future without pandemics—and without the flu—is worth investing in.
Pioneering research
She helped change vaccines forever
Long before most of us heard of mRNA vaccines, this hero saw their potential to save lives.
For most people, the highly effective mRNA COVID vaccines made by Moderna and Pfizer-BioNTech seemed to come out of the blue. But these new vaccines, which were essential to end this pandemic and will likely play a critical role in preventing future pandemics, are the product of decades of painstaking work by researchers.
One of those researchers is Dr. Katalin Karikó, a Hungarian biochemist who long ago saw the potential of mRNA to save lives when few others did.
The daughter of a small-town butcher in Hungary, Karikó knew from a young age that she wanted to become a scientist. She was drawn to biochemistry and developed a particular fascination with messenger RNA, or mRNA, molecules that (among other things) direct the creation of proteins in your body.
Messenger RNA functions as a kind of middleman—it carries the directions for making proteins from your DNA to the factories in your cells where the proteins will be assembled. It’s a bit like the waiter in a restaurant who writes down your order and takes it to the kitchen, where the cooks will make your meal.
In the 1980s, while working on her PhD in her native Hungary, Karikó became convinced that tiny strands of mRNA could be injected into cells to send instructions to the body to make its own medicines. She was interested in developing mRNA treatments for stroke, cancer, and other diseases.
Although vaccines were not the focus of Karikó’s work, other researchers saw that it would be possible to use mRNA to make those as well—for flu, coronaviruses, and maybe even various forms of cancer.
Using mRNA to make vaccines would be a major departure from the way most vaccines work. Many conventional vaccines operate by injecting a weakened or dead form of the virus you’re trying to stop. Your immune system sees the new shapes on the virus, kicks into gear, and builds up immunity. While conventional vaccines have been very effective, it takes years of lab work and clinical studies to make sure that they are safe and will produce a good immune response.
The idea behind mRNA vaccines was quite clever. Since mRNA takes the orders for proteins from the DNA and delivers them to the cooks in your cells’ kitchen, what if we could change those orders in a very targeted way? By teaching your cells to make shapes that match shapes on the actual virus, the vaccine would trigger your immune system without having to introduce the virus itself.
If they could be made, mRNA vaccines would be a huge advance over conventional vaccines. Once you had mapped out all the proteins that make up the virus you wanted to target, you’d identify the one that you want antibodies to grab. Then you’d study the virus’s genetic code to find the instructions for making that protein, and you’d put that code into the vaccine using mRNA. If, later, you wanted to attack a different protein, you’d just change the mRNA. This design process would take at most a few weeks. You would ask the waiter for fries instead of a side salad, and your immune system would do the rest.
There was just one problem: It was only a theory. No one had ever actually made an mRNA vaccine. What’s more, most people in the field thought it was crazy to even try, not least because mRNA is inherently unstable and prone to degrading quickly. Also, cells have evolved to avoid being hijacked by foreign mRNA, and there would need to be a way of getting around this defense system.
Karikó’s interest in mRNA eventually brought her to the U.S. And in 1993, while doing research at the University of Pennsylvania, Karikó and her boss managed a feat that told them they were on to something: They got a human cell to produce a tiny amount of new proteins using a modified version of mRNA that had been altered so it could get past the cell’s defense system. This was a breakthrough, because it meant that if they could expand the production dramatically, they would be able to make a cancer treatment using mRNA.

Dr. Katalin Karikó, who knew from a young age that she wanted to become a scientist, works in her lab, 1985. (personal photo/Katalin Karikó)
Stories of medical discoveries often don’t travel in straight line from breakthrough to lifesaving impact. And Karikó’s story is no different. Karikó’s work lost momentum when her boss left academia for a biotech firm. She no longer had a lab or financial support for her work; although she applied for grant after grant, every application was rejected. In 1995, she had a cancer scare, she was taken off the tenure track at work, and her husband was stuck in Hungary because of a problem with his visa. But Karikó was undeterred.
Then in 1997, she began working with Drew Weissman, a new colleague who came to the University of Pennsylvania with a promising background: He had done a fellowship at NIH under the supervision of Tony Fauci, and he was interested in using Karikó’s work on mRNA to develop vaccines.
Together Karikó and Weissman kept pursuing the idea of working with mRNA that had been engineered in a lab. But they still had to get more mRNA past the cell’s defense systems, a problem that other scientists helped solve. In 1999, a cancer researcher named Pieter Cullis and his colleagues proposed that lipids—basically, tiny bits of fat—could be used to encase and protect a more delicate molecule, such as mRNA. Six years later, working with Cullis, biochemist Ian MacLachlan did it for the first time. The lipid nanoparticles he developed paved the way for the first mRNA vaccines.

At their University of Pennsylvania lab, Dr. Katalin Karikó and her colleague Dr. Drew Weissman helped develop the technology that made mRNA vaccines possible.(University of Pennsylvania Medical School)
As late as 2010, hardly anyone in the federal government or private industry was interested in trying to make vaccines using mRNA. Major pharmaceutical companies had tried and failed, and some scientists felt that mRNA would never trigger enough of a response in the body. But an official at DARPA, the little-known research program for the U.S. military, saw enough promise in the technology that he started funding mRNA vaccines for infectious diseases.
As pioneering as this work was, it didn’t lead immediately to new vaccines. Accomplishing that would be the task of companies dedicated to translating the breakthrough into a product that could be approved and sold; the U.S.-based Moderna and Germany-based CureVac and BioNTech were founded to do just that.
In 2014, Karikó joined BioNTech, which was working on an mRNA vaccine for cancer. Early efforts didn’t work, although a test of a rabies vaccine showed promise. Still, Karikó and her BioNTech colleagues persevered, as did scientists at Moderna. When COVID hit, they immediately set out to make a vaccine for the new virus. It was a good bet.
The notion that mapping a virus’s genome would allow you to create an mRNA vaccine in a matter of weeks proved to be exactly right. In March 2020, just six weeks after scientists sequenced the COVID virus’s genome, Moderna announced that it had identified an mRNA-based candidate and begun making it for clinical trials. On December 31, the mRNA vaccine made by BioNTech in partnership with Pfizer was approved for emergency use by the World Health Organization. When Karikó received the first dose of the vaccine she had done so much to create—a few days before it was officially approved—she wept.
When Dr. Katalin Karikó got her first dose of the vaccine she had done so much to create, she wept. (University of Pennsylvania Medical School)

Dr. Katalin Karikó, Senior Vice President of BioNTech, celebrates with the co-founders of BioNTech after being presented with the German Future Prize award in Berlin, Germany, 2021 for their work on the COVID-19 vaccine. (Picture Aliiance/Getty Images)
When Dr. Katalin Karikó got her first dose of the vaccine she had done so much to create, she wept. (University of Pennsylvania Medical School)
Dr. Katalin Karikó, Senior Vice President of BioNTech, celebrates with the co-founders of BioNTech after being presented with the German Future Prize award in Berlin, Germany, 2021 for their work on the COVID-19 vaccine. (Picture Aliiance/Getty Images)
For all her amazing foresight, I doubt even Dr. Karikó imagined that mRNA vaccines would one day play an essential role in ending a pandemic – and giving us a tool to prevent the next one. And to me, that’s the important lesson of her story: It’s impossible to predict exactly how breakthroughs will shape the future. That’s why it’s critical, if the science makes sense, that we should be willing to bet on crazy sounding ideas and the researchers like Dr. Kariko willing to fight tooth and nail to pursue them. They just might change the world.
Meet more of my heroes in the field
A plan for the world
3 things we can do right now
If we’re going to make COVID-19 the last pandemic, the world needs to get to work right away on these key areas.
When I sat down to write my new book, my goal was to create a concrete list of steps the world could take to prevent the next pandemic. There’s a lot we can and should learn from COVID-19. But I knew that I wanted to focus more on the future instead of the past.
For decades, people told the world to get ready for a pandemic, but hardly anyone made it a priority. Then COVID struck, and stopping it became the most important thing on the global agenda. Governments need to take action now to get ready for the next pathogen, while all of us still remember how awful COVID was (and still is) and feel the urgency of never allowing another one to happen.
If we’re going to make COVID-19 the last pandemic, the world needs to get to work right away on three key areas:
1. Make and deliver better tools.
The story of Katalin Kariko and mRNA vaccines proves that ideas for new tools must often be nurtured and researched, sometimes for decades, before they produce anything of practical value. That’s why step one in any pandemic-prevention plan should be to keep investing in better vaccines, therapeutics, and diagnostics.
You can read more about how new vaccines can be developed and delivered faster here. This includes improving our ability to test and approve new products, as well as scaling up manufacturing capacity and creating better way of delivering vaccines (like microneedle patches) so we can get out lots of doses fast.
On the therapeutics front, it took nearly two years to find effective treatments for COVID. The trajectory of the pandemic would’ve looked a lot different if we had found them sooner. We need to build out the systems that will allow us to make new treatments much faster in the future.
One key step is to create a library of antiviral compounds that are designed to attack common respiratory viruses, so that we can more easily find out if an existing drug will work in the event of an outbreak. We can also take advantage of advances in artificial intelligence and other computational methods. A computer could quickly scan a 3D model of a pathogen to figure out which drugs might be effective against it. It would be able to tell you which drugs look promising, figure out how to improve them, and, if necessary, even design new ones from scratch.
We should also expand incentives for generics manufacturers to create low-cost versions of new drugs. This can be achieved through advance orders on behalf of low- and middle-income countries, which get generic drugmakers to start manufacturing a new drug through advance orders and agreements that allow one company to manufacture a drug invented by another company even while it’s still going through regulatory approvals.
Another area where we need to spark more innovation is in diagnostics. Researchers should keep working on—and funders should keep supporting—high-throughput PCR tests, which have all the benefits of a PCR but are significantly faster at returning results, much cheaper to run, and easier to adapt to a new pathogen. We also need to support work on new types of tests that make it easier to collect samples and turn around results quickly, like better versions of the rapid antigen tests that many of us now take at home for COVID or even handheld devices that health workers can use to easily test people in their community. And testing should be tied to treatment, so if you test positive, you get the medication you need right away.
2. Improve disease monitoring.
Creating the GERM—Global Epidemic Response and Mobilization—team is one of the most important steps we can take to stop the next pandemic. GERM will play a crucial role in virtually every aspect of pandemic prevention, but improving monitoring will be the most significant part of their mandate.
GERM is only one piece of the puzzle, though. Another crucial step is to improve civil registration and vital statistics in the developing world. At a minimum, many low- and middle-income countries need stronger registries of births and deaths, so that GERM can work with local organizations to more easily spot if there’s an unusual pattern worth investigating. Then, building on that foundation, countries should expand into autopsies that use minimally invasive tissue samplings, wastewater surveillance, and other practices.
The world’s disparate disease monitoring systems also need to be integrated so that public health officials can rapidly detect pathogens. Data must be made available in real time, with test results integrated into the public health system so that officials can watch for outbreaks. And in countries like the United States, where testing can be extremely expensive, governments need to make diagnostics cheaper and more accessible to everyone.
Finally, we need to expand our capacity to sequence the genomes of pathogens in order to track new variants. We should double down on investments in projects like the Africa Pathogen Genomics Initiative, a network of labs across the continent that share genomic data with each other, and in new tools that will let us sequence more genomes in more places.
3. Strengthen health systems.
Good health care starts with good health systems. That’s true for basic care, and it’s especially true for pandemic prevention. When a new or deadly pathogen emerges, you need somewhere for sick people to reliably seek treatment. You need health workers who can identify potential threats and the infrastructure to report anything out of the ordinary. And, once a pathogen starts to spread, you need trained professionals who can administer higher level tests, treatments, vaccines, and more.

A nurse carries out PCR testing for COVID-19 on patient in Uganda.
A health worker prepares the COVID-19 vaccine at the Abuja National Hospital in Nigeria
A nurse carries out PCR testing for COVID-19 on patient in Uganda.
A health worker prepares the COVID-19 vaccine at the Abuja National Hospital in Nigeria
The pandemic devastated health systems around the world, but the need in low-income countries is especially acute. The fundamental challenge is that they don’t have the funding, expert capacity, or institutions they need to offer basic health services to all their people, let alone manage a major outbreak. And during the pandemic, the problem got worse, as many rich governments cut foreign aid or took money from work on other diseases and redirected it to COVID.
We need to reverse this trend. A major part of the Gates Foundation’s work has been to help improve health systems—investments that both save lives, end preventable infectious diseases, and pave the way for economic growth. But philanthropy alone cannot close the gap between rich and poor countries. The models for wealthy countries are still Sweden and Norway, who each give at least 0.7 of their GDP in aid. If we’re going to be serious about preventing the next pandemic, we need to not just go back to pre-COVID aid levels but increase investments in strengthening health systems (which will also help shrink the overall health gap between the rich and the poor).
For their part, low- and middle-income countries should focus on health spending that achieves many things at once. For example, hiring more health workers gives you more people who can manage malaria cases, offer HIV testing and treatment, and give public officials unprecedented insight into what’s causing illness and death in their country.
But as COVID made clear, low- and middle-income countries aren’t the only ones that need to strengthen their health systems. There are steps that countries at every income level should consider, like improving primary health care and deciding in advance of a crisis who will oversee what. Governments and donors also need a global forum where they can coordinate action with poor countries.
All of these efforts—new tools, better disease surveillance, and improved health systems—won’t be cheap, but they will save lives and money in the long run. I estimate that, over the next decade, governments combined need to spend $15 to $20 billion per year to develop the tools we need. Strengthening health system will cost the world an extra $30 billion a year, on top of the money we should already be spending to improve health in low income countries.
That sounds like a lot of money until you learn that the International Monetary Fund estimates this pandemic will cost $12.5 trillion over just five years. Think of it like insurance. This is the billions we need to spend in order to save millions of lives and trillions of dollars.

And here’s the best news: Even when we’re not facing an active outbreak, these investments will make people healthier, save lives, and shrink the health gap between the rich and the poor. This is an opportunity to not just stop things from getting worse but to make them better.
We don’t need to surrender to living in perpetual fear of another global catastrophe. But we do need to remain aware of the possibility and be willing to do something about it. I hope the world seizes this moment and invests in the steps needed to make COVID-19 the last pandemic.